Built for More: Uniform Coverage, Higher Yields, and Confident Variant Characterization.
Twist TrueAmp Library Preparation delivers consistent, high quality libraries for NGS target enrichment workflows, especially with challenging low input samples. Powered by Twist’s TrueAmp polymerase and optimized for enzymatic fragmentation, this workflow delivers high conversion efficiency, low GC bias, and balanced coverage, supporting accurate variant characterization across challenging genomic regions.
Built for More: Uniform Coverage, Higher Yields, and Confident Variant Characterization.
Twist TrueAmp Library Preparation delivers consistent, high quality libraries for NGS target enrichment workflows, especially with challenging low input samples. Powered by Twist’s TrueAmp polymerase and optimized for enzymatic fragmentation, this workflow delivers high conversion efficiency, low GC bias, and balanced coverage, supporting accurate variant characterization across challenging genomic regions.
Product data
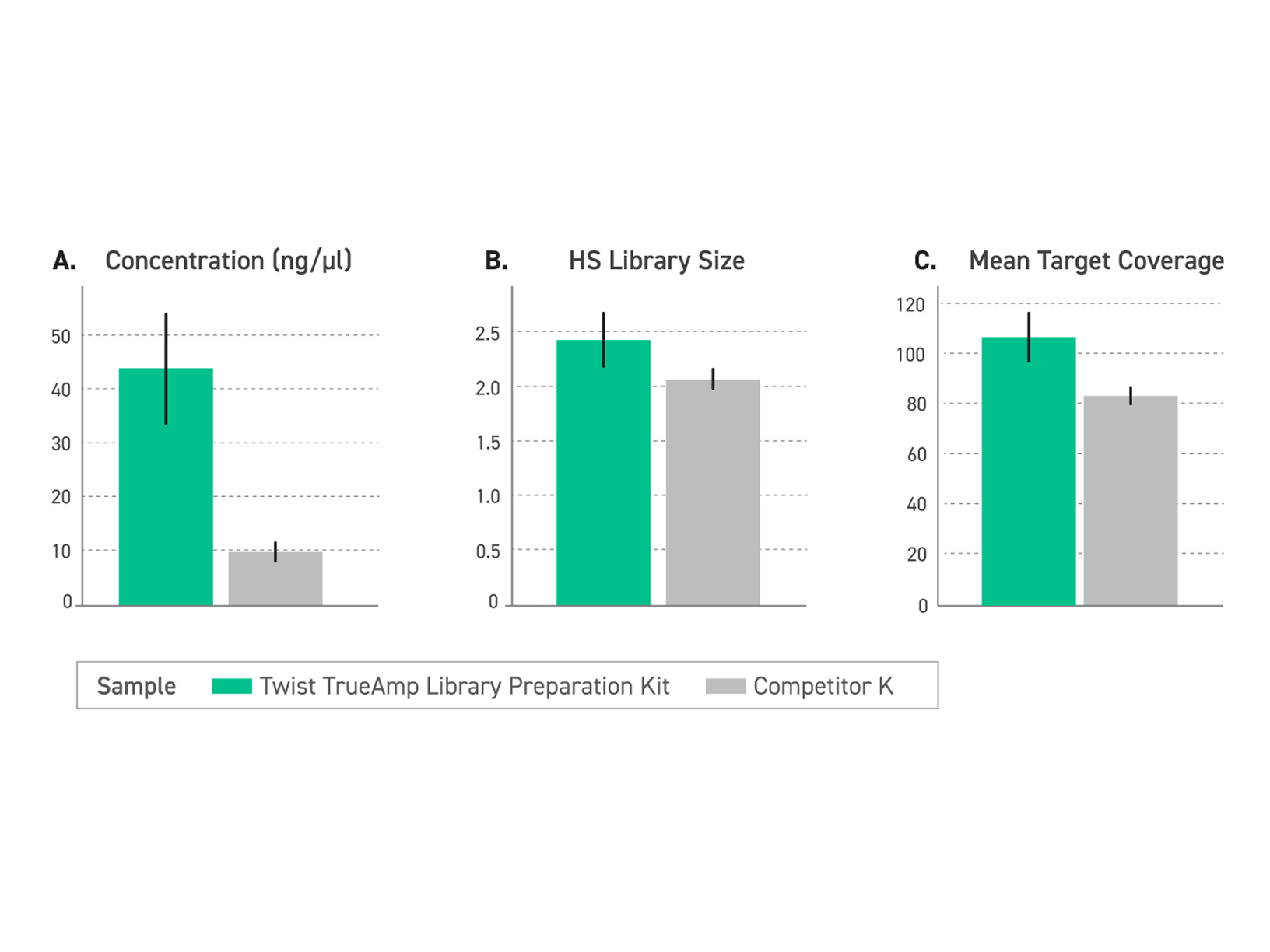
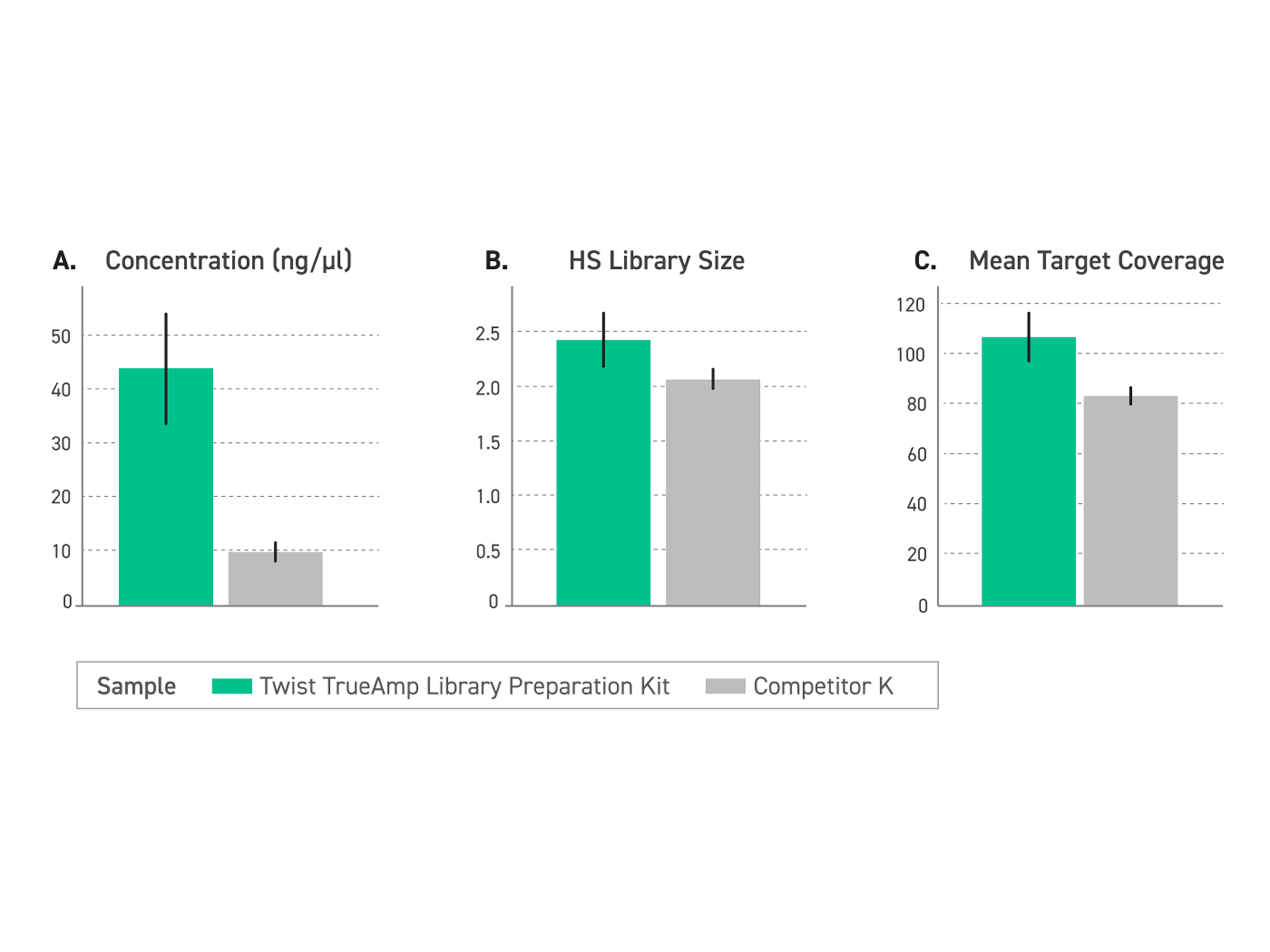
Figure 1. Performance comparison of enriched libraries with low-input FFPE degraded samples (DIN <2.2) between TrueAmp Library Preparation Kit and competitor K kit, demonstrating the optimal solution for challenging sample applications. (A) The Twist TrueAmp Library Preparation Kit generates superior pre-capture library yield, indicative of high library construction and amplification efficiency. (B) The Twist TrueAmp Library Preparation Kit shows higher library complexity when compared with the competitor’s kits. This allows for more unique DNA molecules that are sequenceable in the library, reducing sequencing costs. (C) Achieves higher coverage.
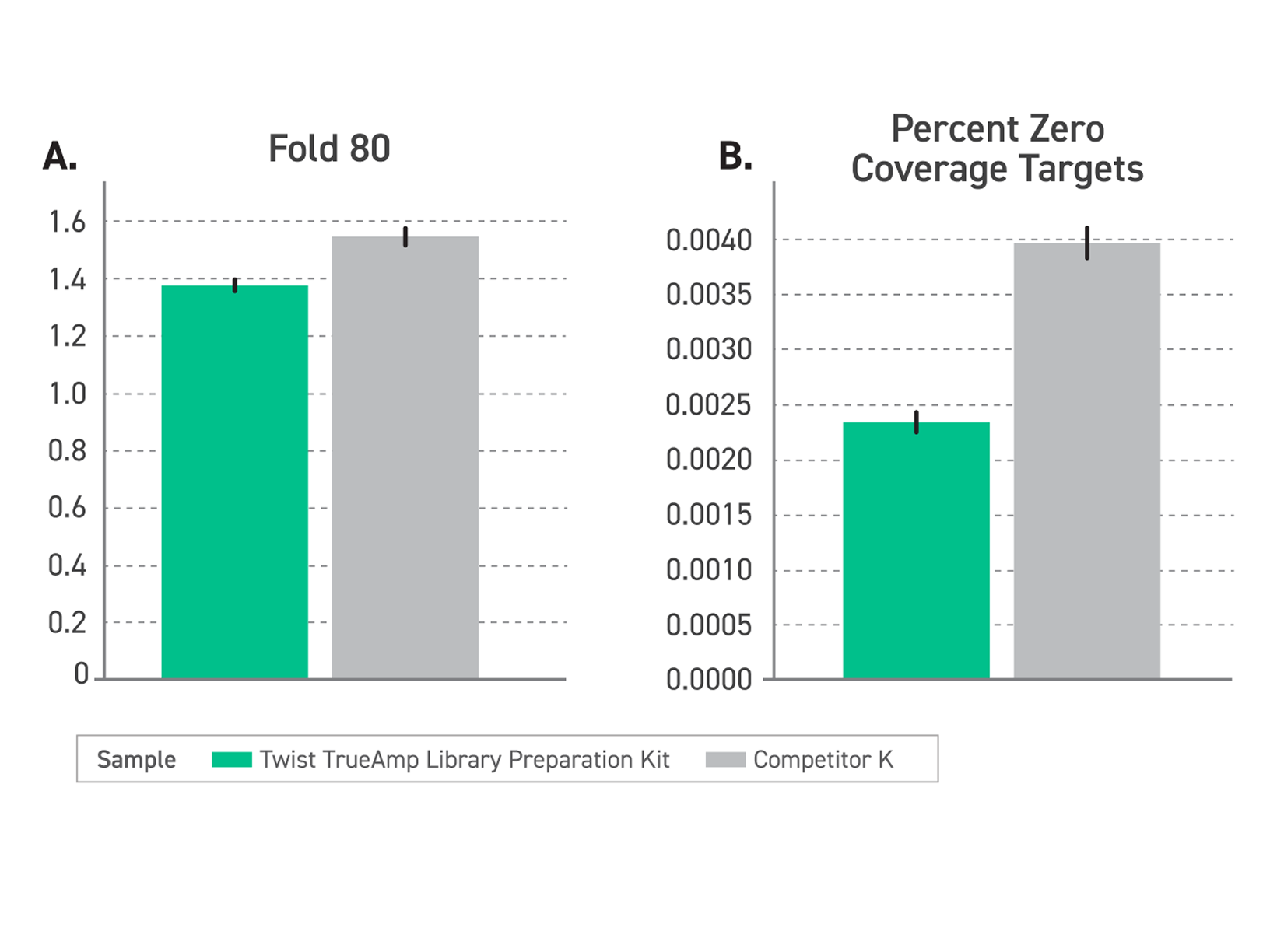
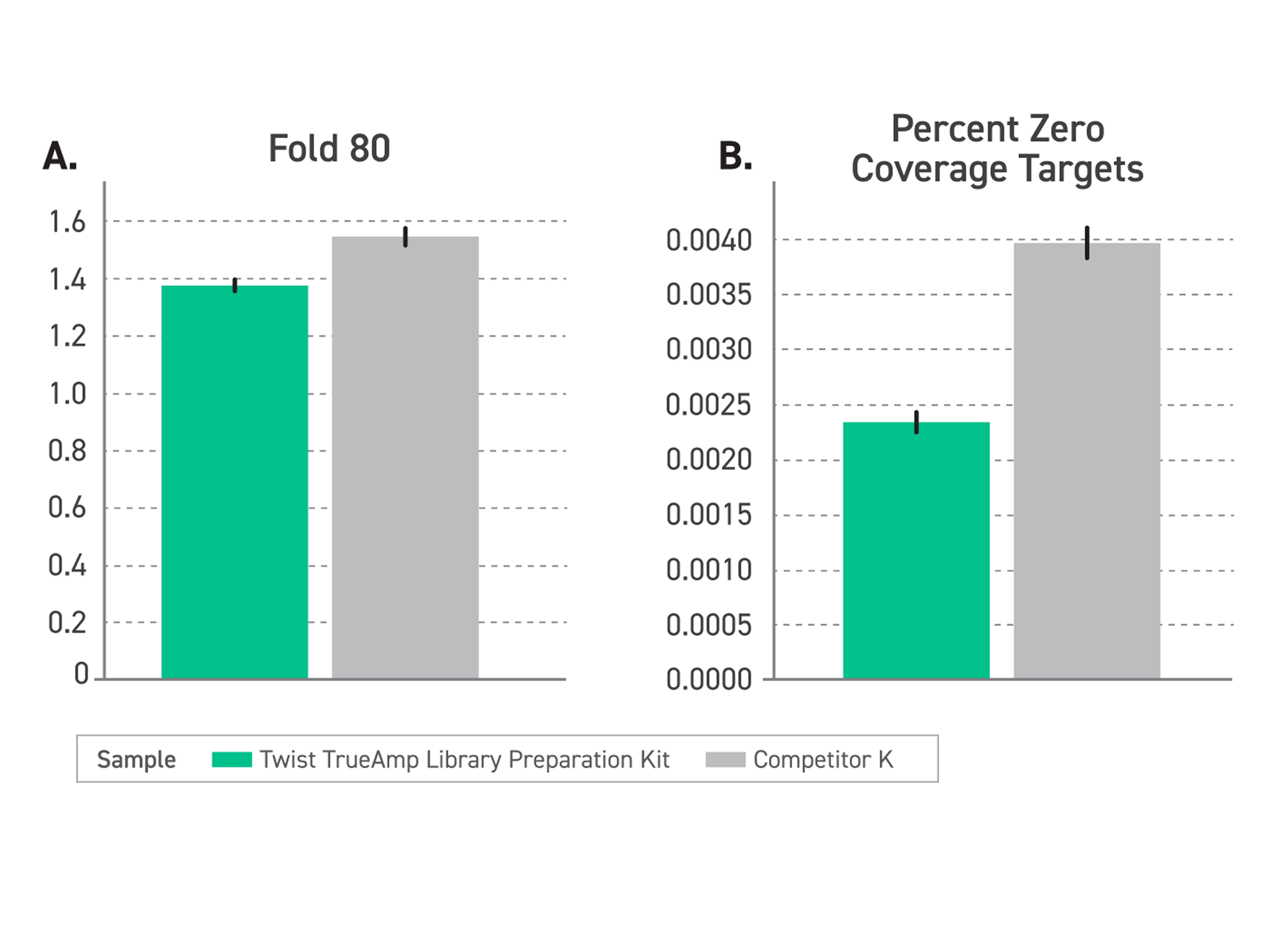
Figure 2. Performance comparison of enriched libraries with low-input FFPE degraded samples (DIN <2.2) between TrueAmp Library Preparation Kit and competitor K kit, demonstrating the optimal solution for challenging sample applications. (A) Delivers excellent coverage uniformity, measured by a lower fold-80 base penalty. (B) Reduced regions with no coverage, measured by Percentage of Zero Coverage Targets.
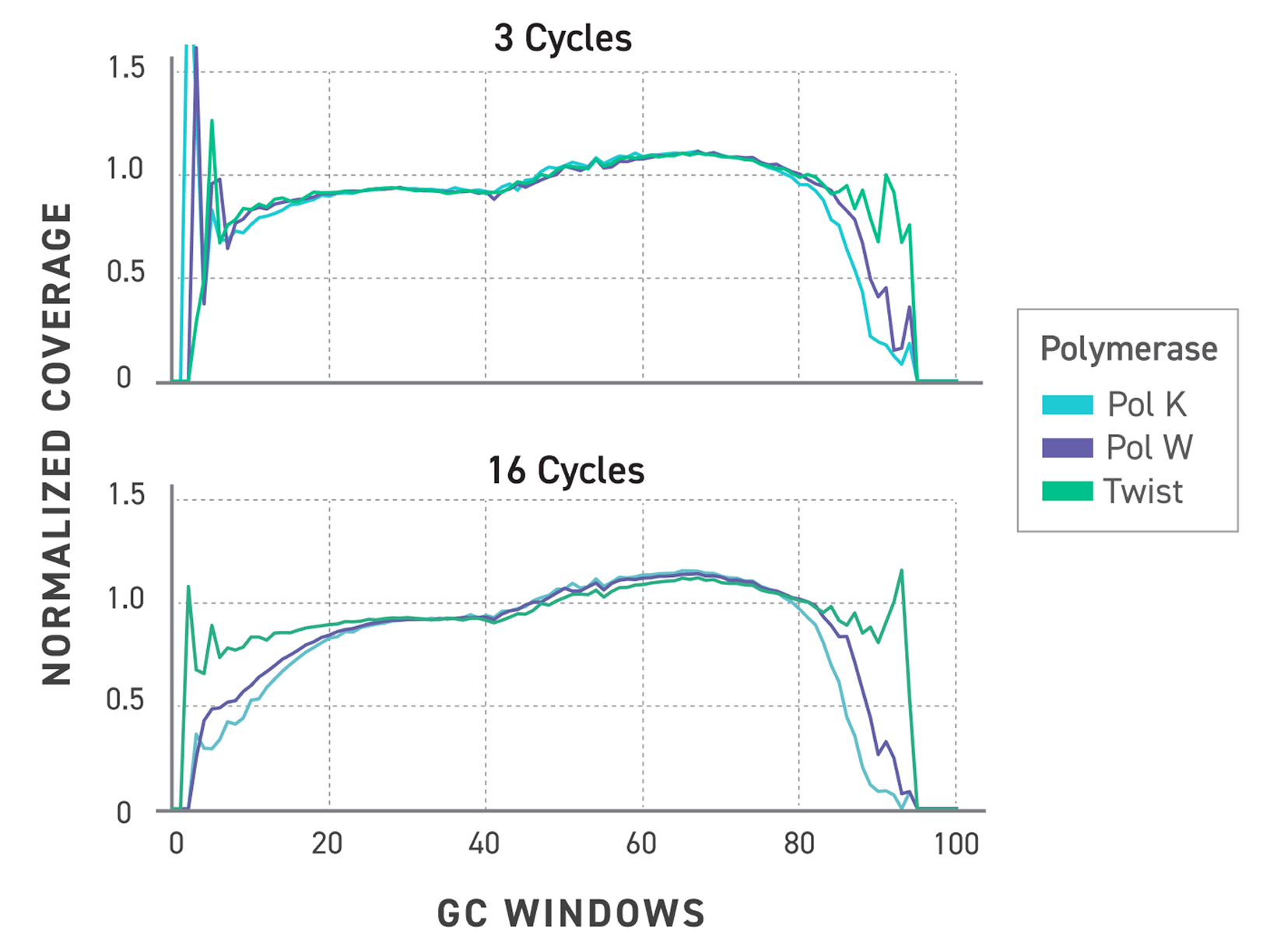
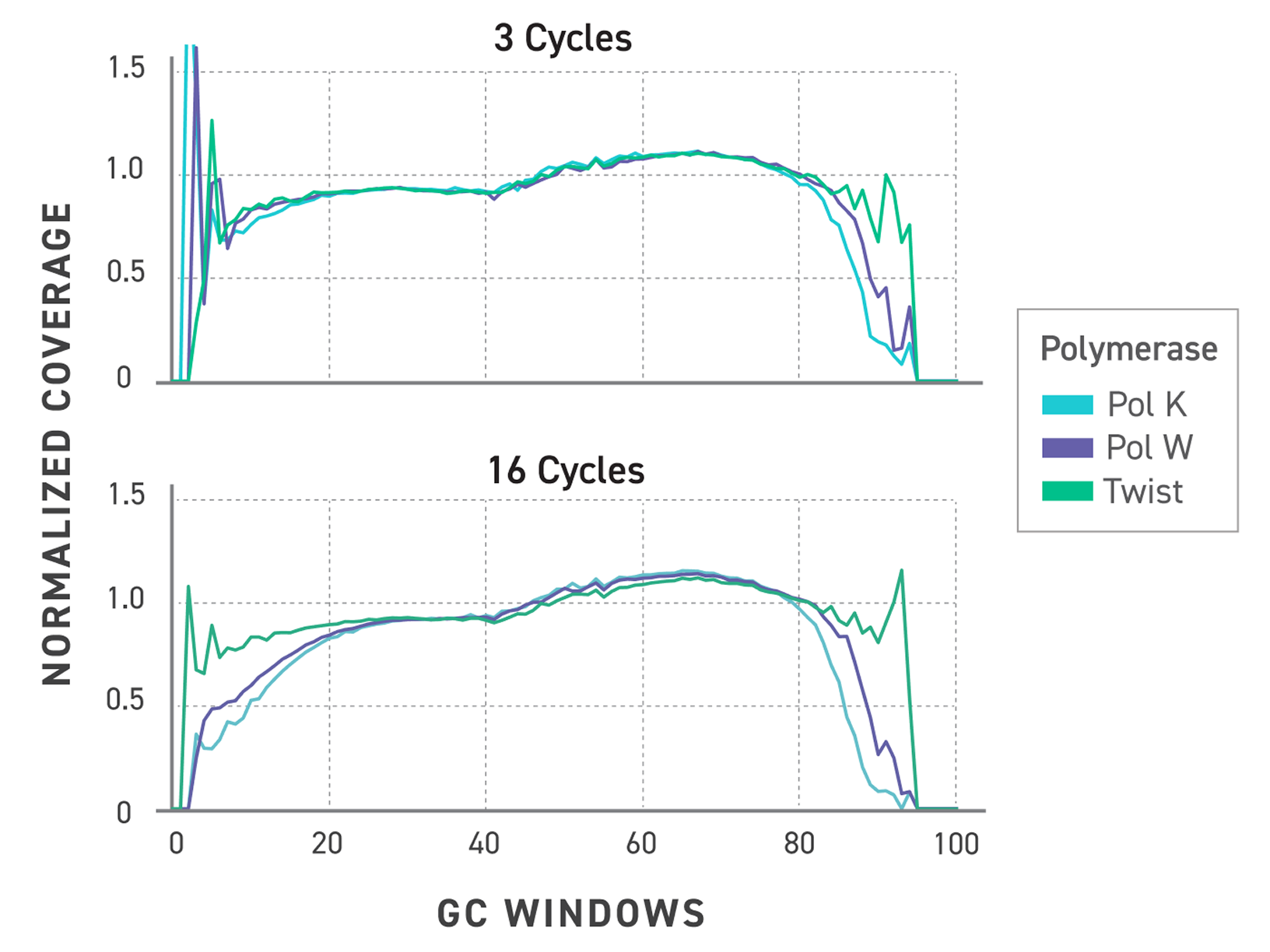
Figure 3. Normalized GC bias trace showing improved coverage of the Twist TrueAmp polymerase. Libraries were prepared with Twist TrueAmp Library Preparation Kit and amplified with different polymerases and cycles.
Upper panel: Normalized coverage against GC window plots comparing polymerases at 3 cycles of PCR.
Lower panel: Normalized coverage against GC window plots comparing polymerases at 16 cycles of PCR.
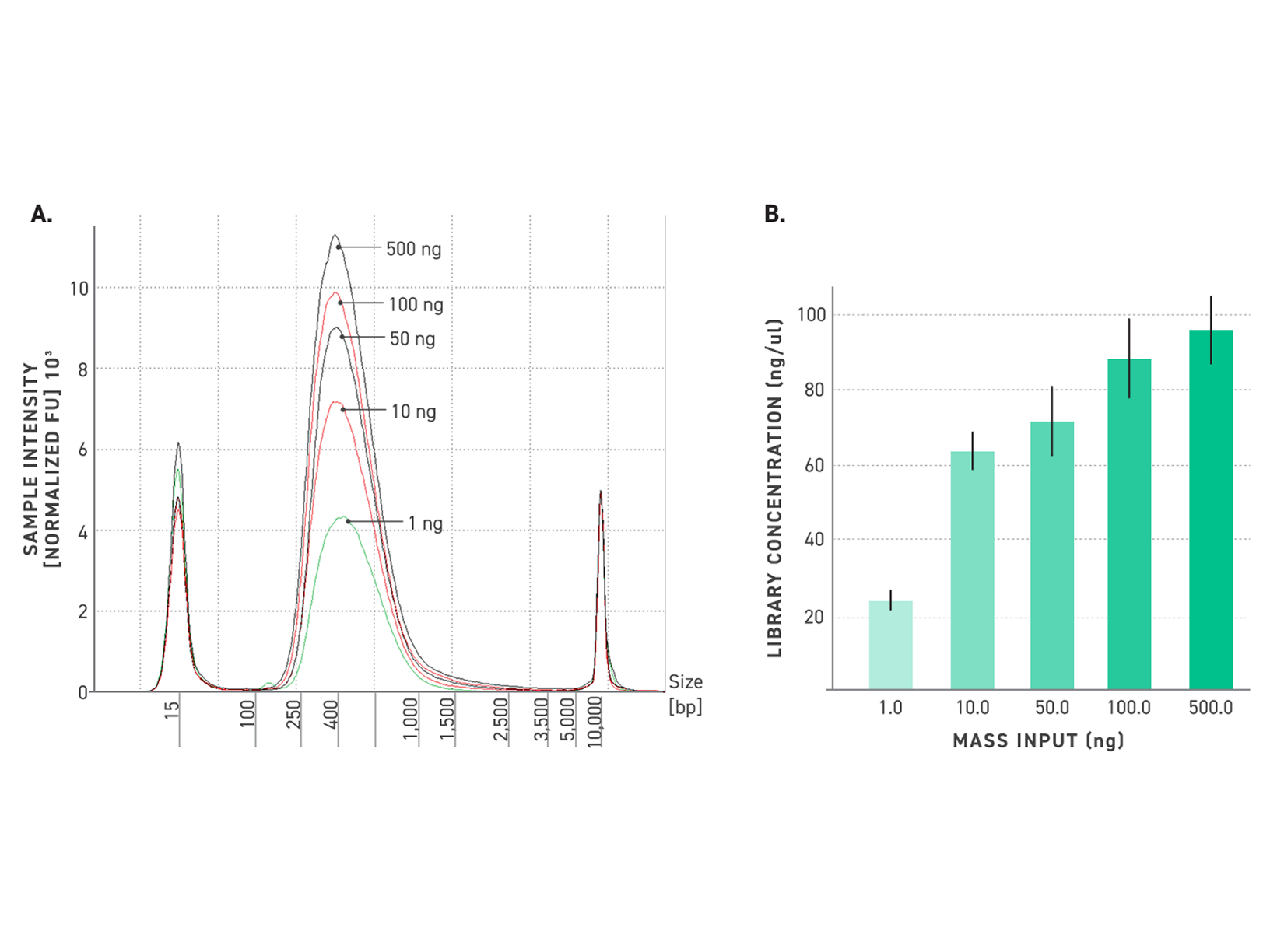
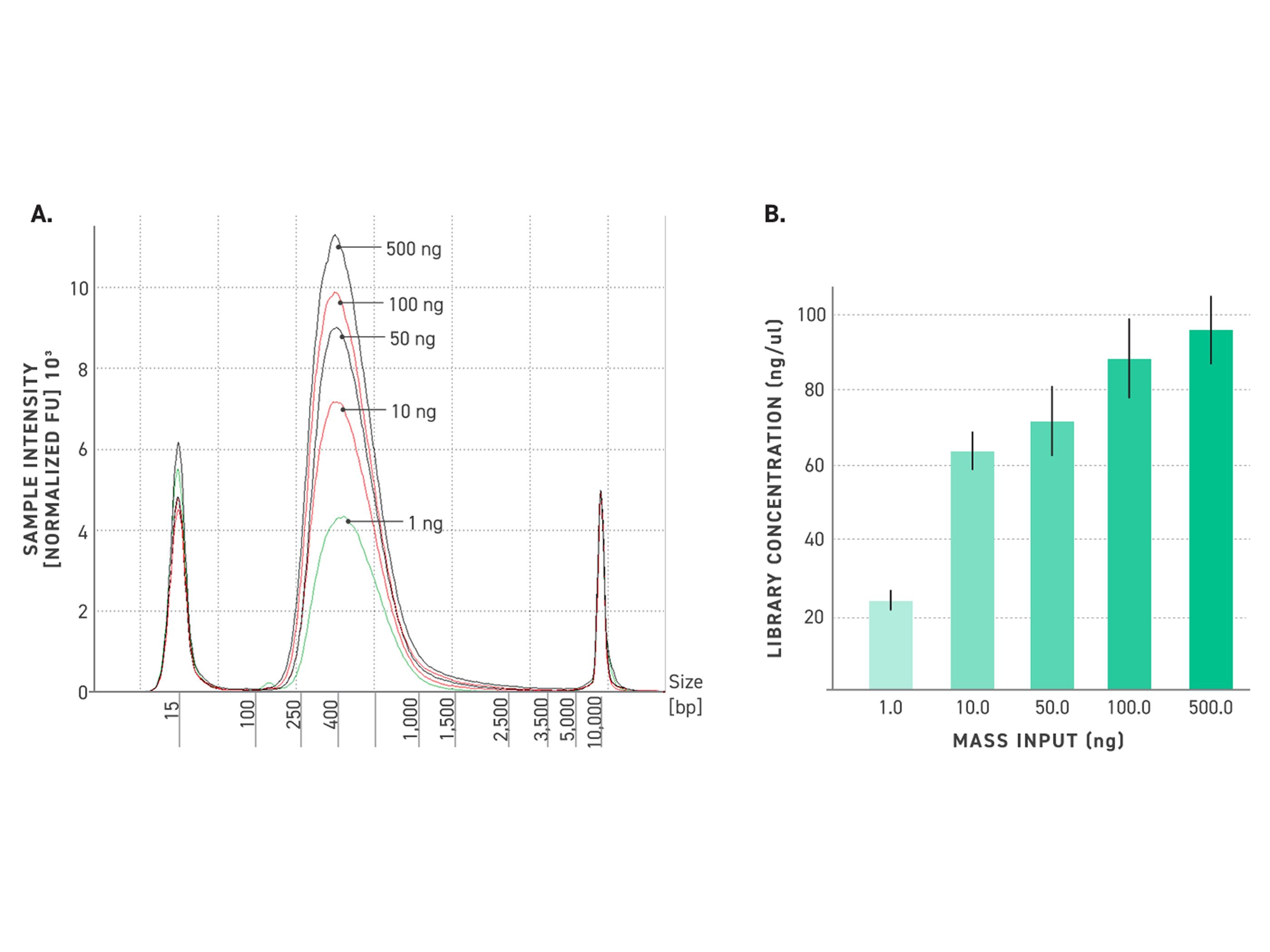
Figure 4. Reliable library size with Twist TrueAmp Library Prep Kit, even from ultra-low inputs.
500 ng, 100 ng, 50 ng, 10 ng and 1 ng (gDNA) were fragmented at 32°C. 3, 5, 6, 8, 10, and 14 cycles of PCR were utilized for amplification, respectively. Samples have been performed in duplicates.
A: Electropherograms of NGS libraries generated with the Twist TrueAmp Library Preparation Kit.
B: Concentration of libraries after amplification for various DNA inputs.
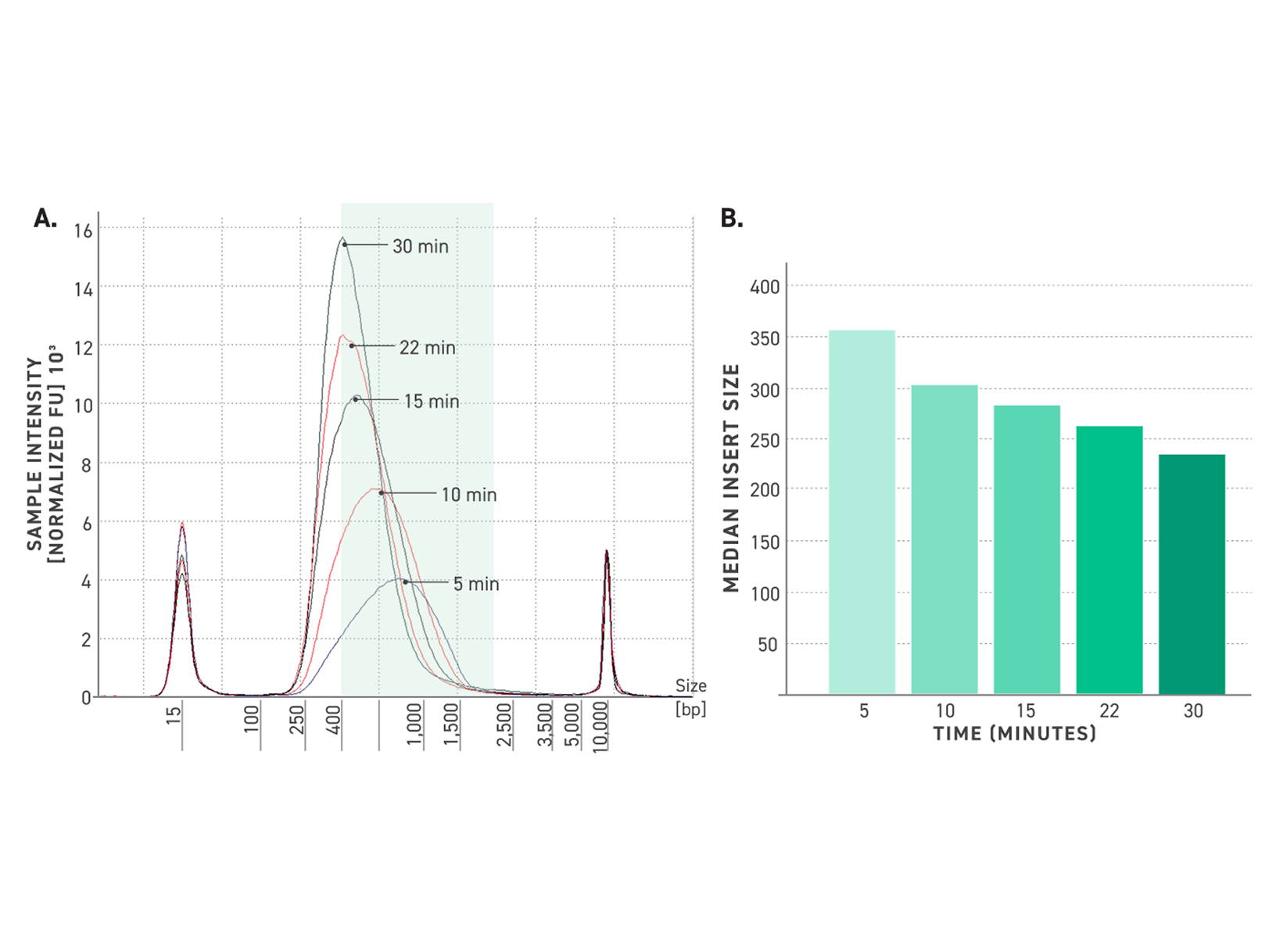
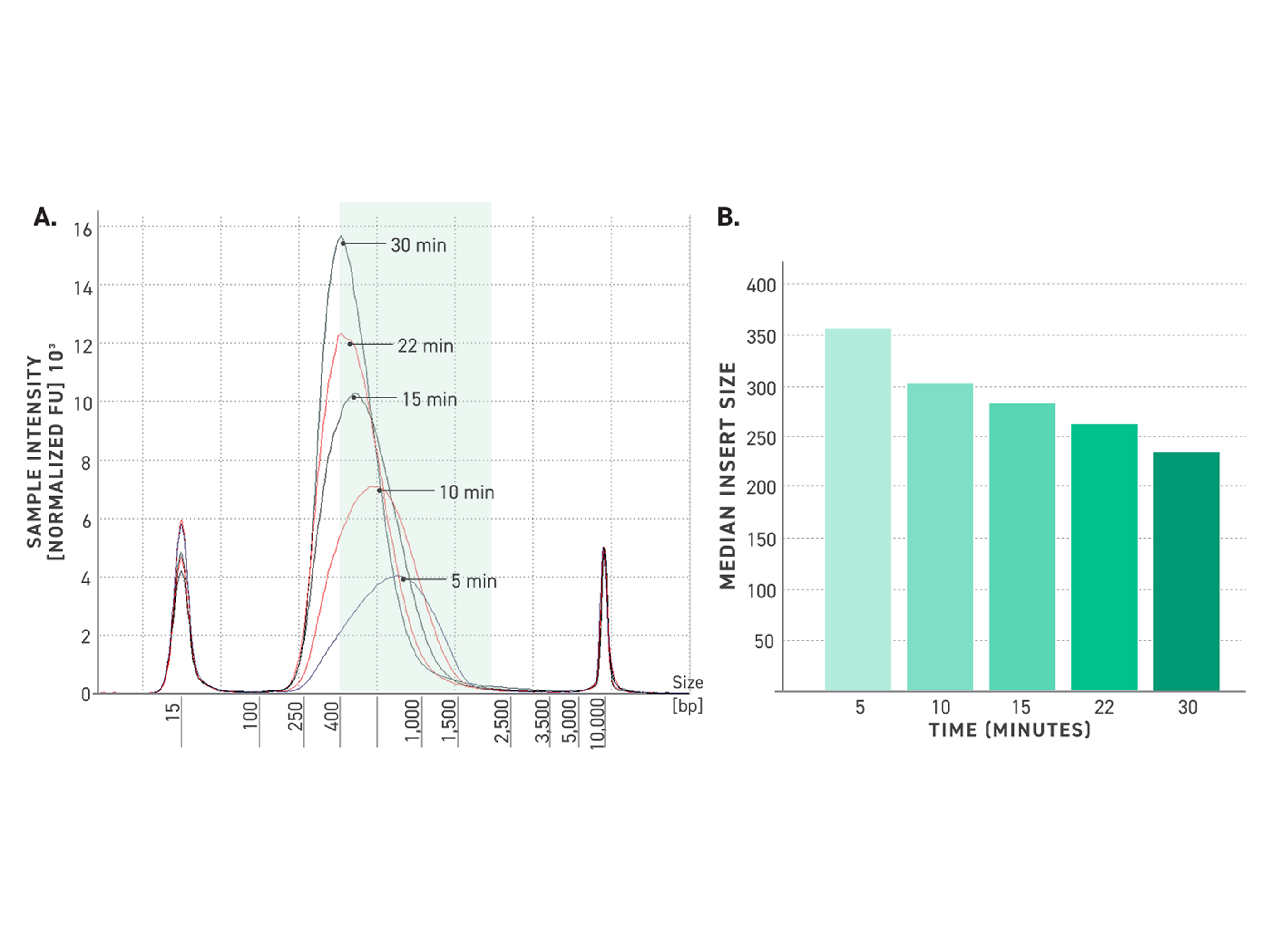
Figure 5. Tunability of Twist TrueAmp Library Prep Kit.
A: Five electropherograms of NGS libraries generated using differing fragmentation times. 50 ng of high-quality gDNA was fragmented for various times at 32°C. 6 cycles of PCR were utilized for amplification.
B: Median insert size vs time. 50 ng of high-quality gDNA was fragmented for various times at 32°C. Amplification was performed using 6 cycles of PCR. Samples were captured using the Twist Exome 2.0 panel.
Product data
How it works
Step 
Enzymatic Fragmentation
Extracted Sample DNA is precisely fragmented producing consistent, tunable insert sizes.
Step 
Adapter Ligation
Optimized Twist ligase maximizes conversion efficiency and minimizes ligation bias.
Step 
Amplification via TrueAmp Polymerase
High fidelity amplification supports high yields in challenging regions and boosts variant detection power.
Product data

Figure 1. Performance comparison of enriched libraries with low-input FFPE degraded samples (DIN <2.2) between TrueAmp Library Preparation Kit and competitor K kit, demonstrating the optimal solution for challenging sample applications. (A) The Twist TrueAmp Library Preparation Kit generates superior pre-capture library yield, indicative of high library construction and amplification efficiency. (B) The Twist TrueAmp Library Preparation Kit shows higher library complexity when compared with the competitor’s kits. This allows for more unique DNA molecules that are sequenceable in the library, reducing sequencing costs. (C) Achieves higher coverage.

Figure 2. Performance comparison of enriched libraries with low-input FFPE degraded samples (DIN <2.2) between TrueAmp Library Preparation Kit and competitor K kit, demonstrating the optimal solution for challenging sample applications. (A) Delivers excellent coverage uniformity, measured by a lower fold-80 base penalty. (B) Reduced regions with no coverage, measured by Percentage of Zero Coverage Targets.

Figure 3. Normalized GC bias trace showing improved coverage of the Twist TrueAmp polymerase. Libraries were prepared with Twist TrueAmp Library Preparation Kit and amplified with different polymerases and cycles.
Upper panel: Normalized coverage against GC window plots comparing polymerases at 3 cycles of PCR.
Lower panel: Normalized coverage against GC window plots comparing polymerases at 16 cycles of PCR.

Figure 4. Reliable library size with Twist TrueAmp Library Prep Kit, even from ultra-low inputs.
500 ng, 100 ng, 50 ng, 10 ng and 1 ng (gDNA) were fragmented at 32°C. 3, 5, 6, 8, 10, and 14 cycles of PCR were utilized for amplification, respectively. Samples have been performed in duplicates.
A: Electropherograms of NGS libraries generated with the Twist TrueAmp Library Preparation Kit.
B: Concentration of libraries after amplification for various DNA inputs.

Figure 5. Tunability of Twist TrueAmp Library Prep Kit.
A: Five electropherograms of NGS libraries generated using differing fragmentation times. 50 ng of high-quality gDNA was fragmented for various times at 32°C. 6 cycles of PCR were utilized for amplification.
B: Median insert size vs time. 50 ng of high-quality gDNA was fragmented for various times at 32°C. Amplification was performed using 6 cycles of PCR. Samples were captured using the Twist Exome 2.0 panel.
Product data
How it works
Step 
Enzymatic Fragmentation
Extracted Sample DNA is precisely fragmented producing consistent, tunable insert sizes.

Step 
Adapter Ligation
Optimized Twist ligase maximizes conversion efficiency and minimizes ligation bias.

Step 
Amplification via TrueAmp Polymerase
High fidelity amplification supports high yields in challenging regions and boosts variant detection power.

Highlighted resources
Highlighted resources
Related Workflows and Applications
Target enrichment workflows with Twist deliver scalable, low bias, and highly uniform coverage across large and complex genomic regions; enabling broader discovery, higher sensitivity, and greater flexibility than amplicon or array based methods.
Twist offers several tools to support research into liquid biopsies and early cancer characterization.
Related Workflows and Applications
Target enrichment workflows with Twist deliver scalable, low bias, and highly uniform coverage across large and complex genomic regions; enabling broader discovery, higher sensitivity, and greater flexibility than amplicon or array based methods.
Twist offers several tools to support research into liquid biopsies and early cancer characterization.