Minimal Residual Disease (MRD) is the presence of a small number of malignant cancer cells that remain in the body after successful therapeutic intervention. MRD can persist at low levels in patients who may be in remission or not show any phenotypic symptoms. Detectable levels of MRD are a risk factor for recurrence, as the remaining cancer cells in the body can metastasize and proliferate over time. Although conventional surveillance technologies, such as radiographic imaging, are highly sensitive methods, the number of remaining cancer cells may be so small that they cannot be detected until symptoms of recurrence appear.
Monitoring MRD can be achieved with liquid biopsy, which enables diagnostic testing of blood or other bodily fluids. This approach has the potential to detect residual cancer cells earlier than standard surveillance methods, allowing for earlier therapeutic intervention. To do this, circulating tumor DNA (ctDNA) is utilized as a biomarker for liquid biopsy MRD testing. A common approach is to generate a variant profile of a patient’s primary tumor through whole-genome or whole-exome sequencing just prior to therapy. A subset of patient-specific variants is then selected from this primary tumor and a small target enrichment panel (MRD panel) is designed against these variants of interest. After treatment, repeated MRD testing will be used to monitor the disease recurrence of the patient at set time-intervals, so that a re-appearance of the selected ctDNA biomarkers in the blood can be evaluated.
The Twist Bioscience MRD Rapid 500 Panel is a scalable target enrichment solution for monitoring minimal residual disease that leverages Twist’s silicon based DNA synthesis platform to design and manufacture capture panels for personalized medicine. Twist’s proprietary design algorithms and a streamlined manufacturing pipeline enable design automation, synthesis, and rapid turnaround time of as few as 6 business days from the time of order. Cast a broader net to fully characterize adjuvant response at a genomic level with MRD panels ranging from 50 to 500 probes per panel and are compatible with Twist’s existing NGS library preparation and hybrid capture workflow.




Minimal Residual Disease (MRD) is the presence of a small number of malignant cancer cells that remain in the body after successful therapeutic intervention. MRD can persist at low levels in patients who may be in remission or not show any phenotypic symptoms. Detectable levels of MRD are a risk factor for recurrence, as the remaining cancer cells in the body can metastasize and proliferate over time. Although conventional surveillance technologies, such as radiographic imaging, are highly sensitive methods, the number of remaining cancer cells may be so small that they cannot be detected until symptoms of recurrence appear.
Monitoring MRD can be achieved with liquid biopsy, which enables diagnostic testing of blood or other bodily fluids. This approach has the potential to detect residual cancer cells earlier than standard surveillance methods, allowing for earlier therapeutic intervention. To do this, circulating tumor DNA (ctDNA) is utilized as a biomarker for liquid biopsy MRD testing. A common approach is to generate a variant profile of a patient’s primary tumor through whole-genome or whole-exome sequencing just prior to therapy. A subset of patient-specific variants is then selected from this primary tumor and a small target enrichment panel (MRD panel) is designed against these variants of interest. After treatment, repeated MRD testing will be used to monitor the disease recurrence of the patient at set time-intervals, so that a re-appearance of the selected ctDNA biomarkers in the blood can be evaluated.
The Twist Bioscience MRD Rapid 500 Panel is a scalable target enrichment solution for monitoring minimal residual disease that leverages Twist’s silicon based DNA synthesis platform to design and manufacture capture panels for personalized medicine. Twist’s proprietary design algorithms and a streamlined manufacturing pipeline enable design automation, synthesis, and rapid turnaround time of as few as 6 business days from the time of order. Cast a broader net to fully characterize adjuvant response at a genomic level with MRD panels ranging from 50 to 500 probes per panel and are compatible with Twist’s existing NGS library preparation and hybrid capture workflow.




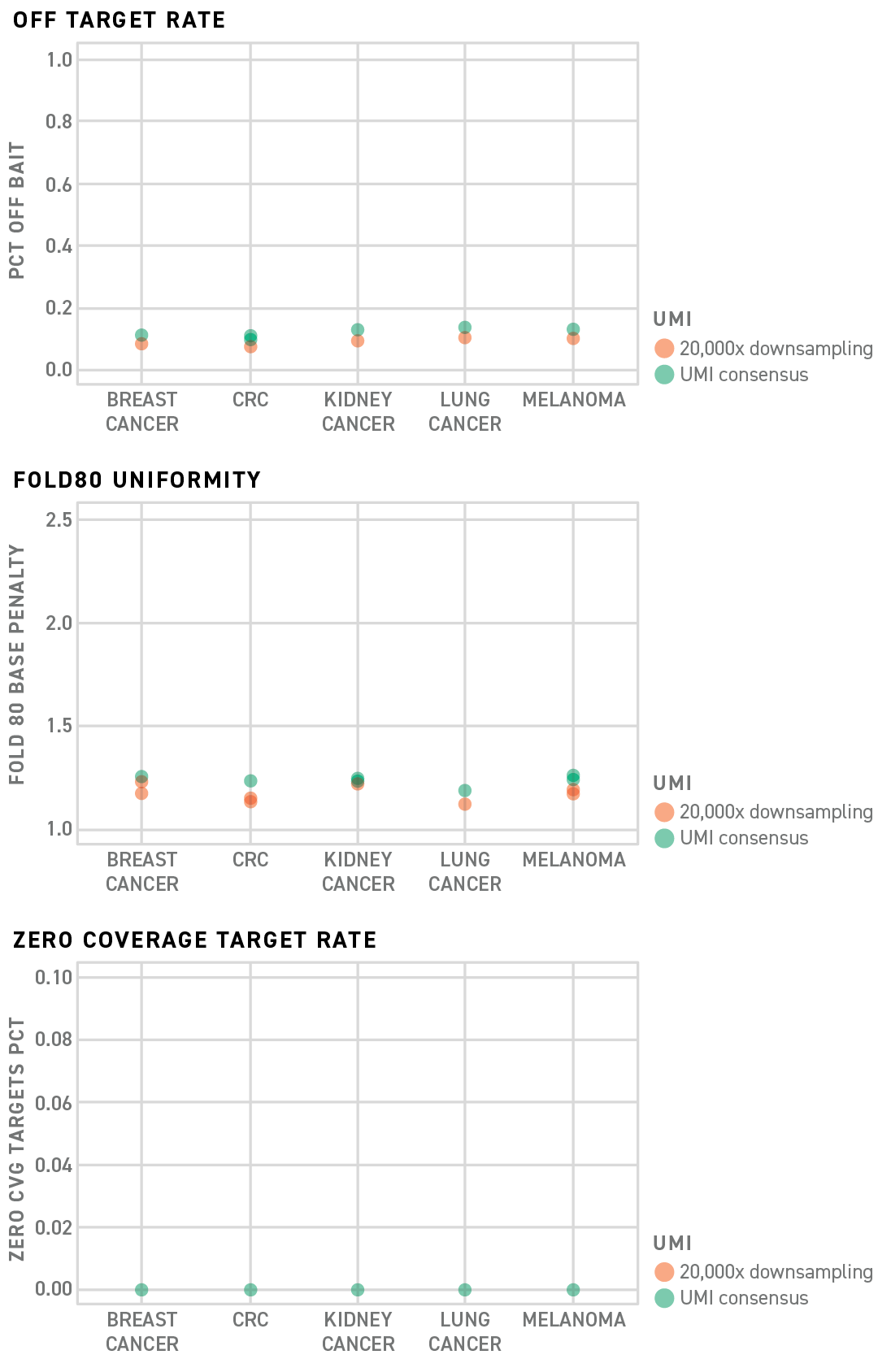
Twist MRD Rapid 500 Panels make the most of your sequencing data by providing industry leading uniform capture technology and streamlined design process. Save on overall sequencing costs with exceptional off target and fold 80 capture performance rates. Monitor all variants of interest with coverage over each of your targets.
Twist has designed five 200 probe MRD panels which specifically target somatic variants found in Breast, Lung, CRC, Melanoma and Renal Cell Carcinoma. In addition, Twist has leveraged its established mechanical library preparation kit with the Twist UMI Adapter System and a newly optimized target enrichment protocol to further improve the MRD panel performance.
Results show that the upgraded system dramatically improves small panel performance that can reduce the off-target rates in each panel to as low as 10–15% with uniform coverage across all targets of interest.
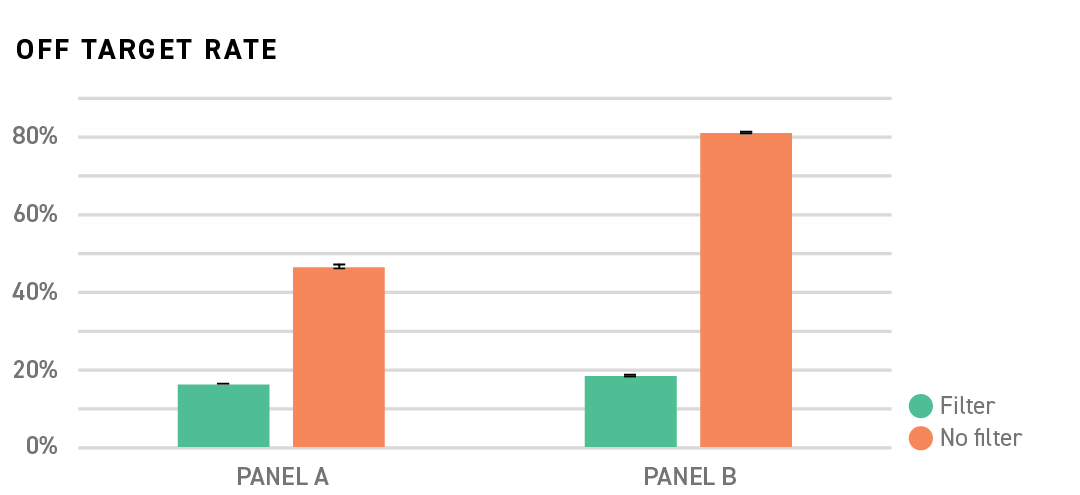
Capture panel performance, particularly panels with few probes, is dependent on the specificity of the probe sequences to the target regions in the genome. Probe species overlapping repetitive elements located throughout the genome can hybridize not only to regions of interest but also many other, unwanted, library fragments resulting in wasted sequencing.
Twist MRD Rapid 500 Panels are designed using proprietary filtering algorithms that will screen probes from a panel if they are predicted to result in increased off target sequencing data. Probes with overlapping repetitive elements can cause unpredictable off target events. Without repetitive filters, as shown in the figure below, the off target rate could be moderate (PanelA No filter) or high (PanelB No filter). Whereas, by filtering out the problematic probes, the off target rates are consistently low (PanelA Filter and PanelB Filter).
Twist MRD Rapid 500 Panels make the most of your sequencing data by providing industry leading uniform capture technology and streamlined design process. Save on overall sequencing costs with exceptional off target and fold 80 capture performance rates. Monitor all variants of interest with coverage over each of your targets.
Twist has designed five 200 probe MRD panels which specifically target somatic variants found in Breast, Lung, CRC, Melanoma and Renal Cell Carcinoma. In addition, Twist has leveraged its established mechanical library preparation kit with the Twist UMI Adapter System and a newly optimized target enrichment protocol to further improve the MRD panel performance.
Results show that the upgraded system dramatically improves small panel performance that can reduce the off-target rates in each panel to as low as 10–15% with uniform coverage across all targets of interest.

Capture panel performance, particularly panels with few probes, is dependent on the specificity of the probe sequences to the target regions in the genome. Probe species overlapping repetitive elements located throughout the genome can hybridize not only to regions of interest but also many other, unwanted, library fragments resulting in wasted sequencing.
Twist MRD Rapid 500 Panels are designed using proprietary filtering algorithms that will screen probes from a panel if they are predicted to result in increased off target sequencing data. Probes with overlapping repetitive elements can cause unpredictable off target events. Without repetitive filters, as shown in the figure below, the off target rate could be moderate (PanelA No filter) or high (PanelB No filter). Whereas, by filtering out the problematic probes, the off target rates are consistently low (PanelA Filter and PanelB Filter).

105878
Twist MRD Rapid 500 panel, 12 testProduct Name
MRD Rapid 500 Panel, 12 TestsMinimum number of probes
50Maximum number of probes
500Supported Input File Format
Target BED File Template (see Resources)Maximum number of panels per order
150Quality Control
qPCRDelivery Method
Matrix TubesSupported Design Genome Assemblies
hg19, hg38Turnaround Time
Panels shipped within 6 business days105878
Twist MRD Rapid 500 panel, 12 testProduct Name
MRD Rapid 500 Panel, 12 TestsMinimum number of probes
50Maximum number of probes
500Supported Input File Format
Target BED File Template (see Resources)Maximum number of panels per order
150Quality Control
qPCRDelivery Method
Matrix TubesSupported Design Genome Assemblies
hg19, hg38Turnaround Time
Panels shipped within 6 business days
Poster
High sensitivity detection of specific ultra low-frequency somatic mutations for MRD monitoring
Poster
High sensitivity detection of specific ultra low-frequency somatic mutations for MRD monitoring