High Sensitivity Synthetic Reference Material for Liquid Biopsy Assay Development and Process Control
The Twist cfDNA Pan-Cancer Reference Standard v2 is a comprehensive tool created to assist researchers in both the development and quality monitoring of clinically relevant variants for developing NGS-based assays. Additionally, researchers can utilize these reference standards to define two essential analytical parameters: the Limit of Detection (LoD) and the Limit of Blank (LoB) for their assays. Composed of wild-type (WT) background cell-free DNA (cfDNA) from a cell-line and synthetic oligos carrying mutant alleles, this reference material offers the precision and sensitivity needed for researching liquid biopsy assay development.
Broad Variant Coverage
The Twist cfDNA Pan-Cancer Reference Standard v2 offers an extensive array of circulating tumor DNA (ctDNA) variants. These variants closely mirror the size and distribution of cfDNA fragments, which can make it an indispensable tool for accurately simulating real-world scenarios. It comprises synthetically-printed variants, including single nucleotide variants (SNVs), insertion-deletions (INDELs), and structural variants (SVs).
Comprehensive Variant Sites
With over 400 variant sites spanning 84 genes, including literature-curated, clinically-relevant variants, this reference standard promotes a comprehensive approach to cfDNA analysis. It empowers researchers to detect a wide range of genetic variations associated with cancer.
Empower LoD and LoB Establishment
The Twist cfDNA Pan-Cancer Reference Standard v2 is meticulously designed to assist in determining the analytical Limit of Detection (LoD) and the Limit of Blank (LoB) of your assays. This critical capability can enable you to define the lowest detectable levels of target variants and establish a baseline for assay sensitivity.
Low Background Error Rate
Comparative analyses demonstrate that the Twist cfDNA Pan-Cancer Reference Standard v2 exhibits a significantly lower error rate in comparison to its predecessor (v1) and other competing products.
Compatibility and Versatility
The Twist cfDNA Pan-Cancer Reference Standard v2 seamlessly integrates with Twist’s suite of target enrichment systems and custom capture panels. The Twist cfDNA Pan-Cancer Reference Standard v2 Set consists of 7 individual tubes with 300 ng per tube for each of the 7 different VAFs: 0% (WT), 0.1%, 0.25%, 0.5%, 1%, 2%, 5%. Twist also offers each individual VAF in a smaller 600ng and larger 3ug configurations.
*For research use only. Not for use in any diagnostic or clinical procedures.
High Sensitivity Synthetic Reference Material for Liquid Biopsy Assay Development and Process Control
The Twist cfDNA Pan-Cancer Reference Standard v2 is a comprehensive tool created to assist researchers in both the development and quality monitoring of clinically relevant variants for developing NGS-based assays. Additionally, researchers can utilize these reference standards to define two essential analytical parameters: the Limit of Detection (LoD) and the Limit of Blank (LoB) for their assays. Composed of wild-type (WT) background cell-free DNA (cfDNA) from a cell-line and synthetic oligos carrying mutant alleles, this reference material offers the precision and sensitivity needed for researching liquid biopsy assay development.
Broad Variant Coverage
The Twist cfDNA Pan-Cancer Reference Standard v2 offers an extensive array of circulating tumor DNA (ctDNA) variants. These variants closely mirror the size and distribution of cfDNA fragments, which can make it an indispensable tool for accurately simulating real-world scenarios. It comprises synthetically-printed variants, including single nucleotide variants (SNVs), insertion-deletions (INDELs), and structural variants (SVs).
Comprehensive Variant Sites
With over 400 variant sites spanning 84 genes, including literature-curated, clinically-relevant variants, this reference standard promotes a comprehensive approach to cfDNA analysis. It empowers researchers to detect a wide range of genetic variations associated with cancer.
Empower LoD and LoB Establishment
The Twist cfDNA Pan-Cancer Reference Standard v2 is meticulously designed to assist in determining the analytical Limit of Detection (LoD) and the Limit of Blank (LoB) of your assays. This critical capability can enable you to define the lowest detectable levels of target variants and establish a baseline for assay sensitivity.
Low Background Error Rate
Comparative analyses demonstrate that the Twist cfDNA Pan-Cancer Reference Standard v2 exhibits a significantly lower error rate in comparison to its predecessor (v1) and other competing products.
Compatibility and Versatility
The Twist cfDNA Pan-Cancer Reference Standard v2 seamlessly integrates with Twist’s suite of target enrichment systems and custom capture panels. The Twist cfDNA Pan-Cancer Reference Standard v2 Set consists of 7 individual tubes with 300 ng per tube for each of the 7 different VAFs: 0% (WT), 0.1%, 0.25%, 0.5%, 1%, 2%, 5%. Twist also offers each individual VAF in a smaller 600ng and larger 3ug configurations.
*For research use only. Not for use in any diagnostic or clinical procedures.
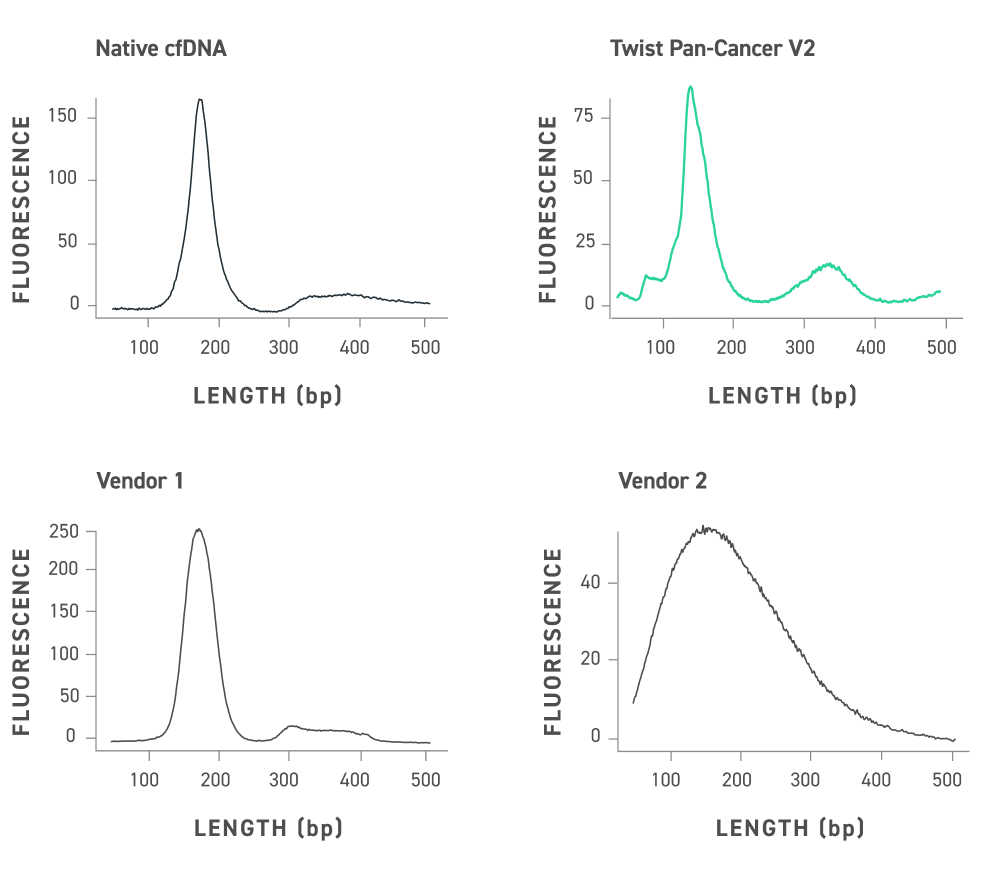
DNA fragment analysis of the Twist cfDNA Pan-cancer Reference Standard v2, native cfDNA and competitor reference standards equalized for peak maximum height. Twist standards have a size distribution that emulates native cfDNA including a prominent mononucleosomal peak and dinucleosomal peak.
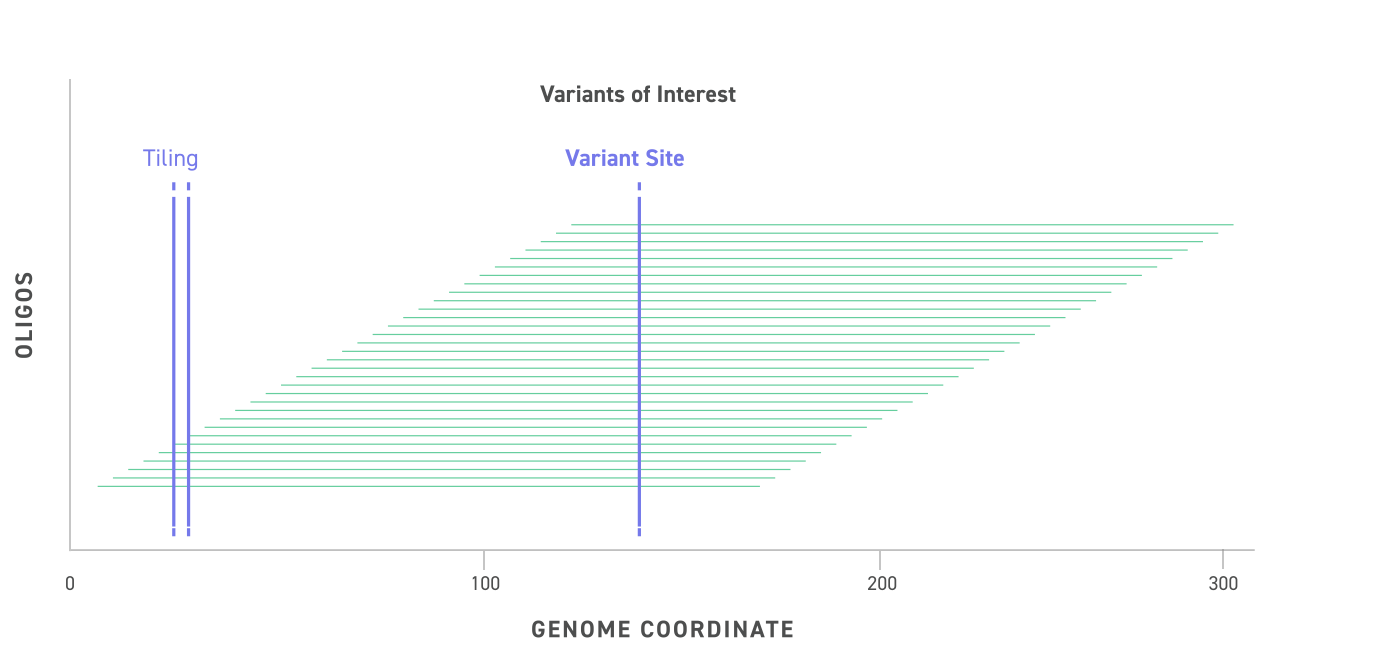
Variant DNA molecules tile across the genomic locus of the variant (purple dashed line with star) with extensive overlap, providing high coverage, fragment diversity, and location diversity of the variant site relative to the termini of the DNA molecule.
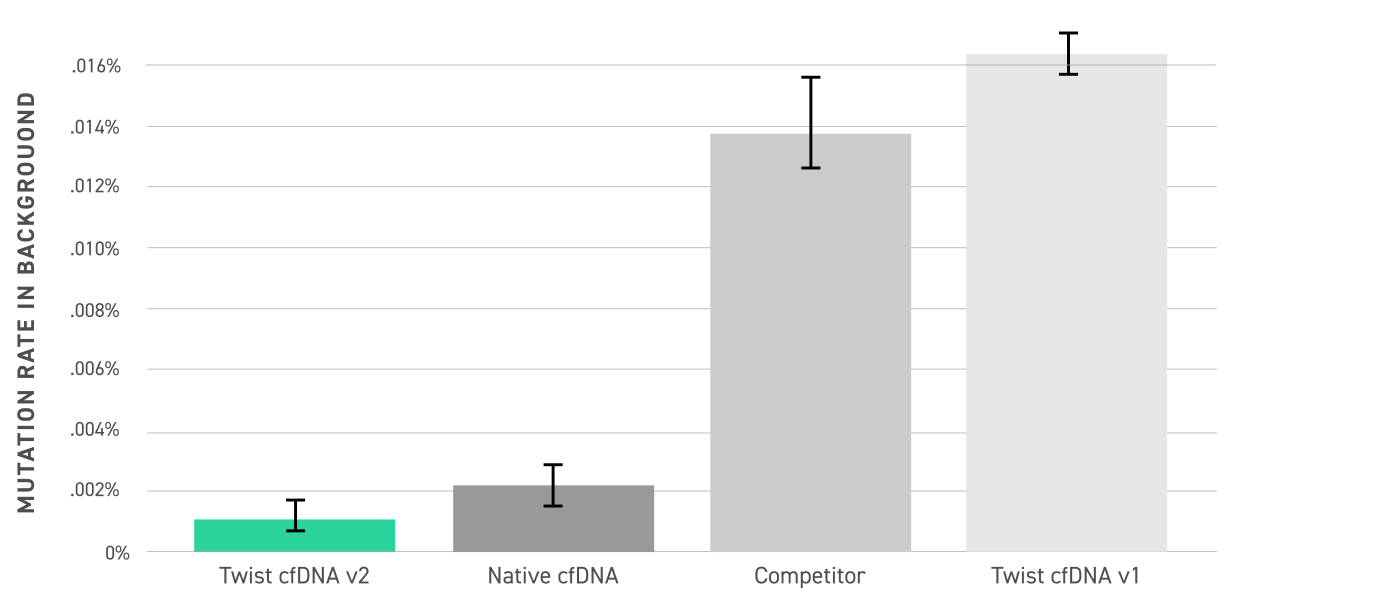
Analysis of background mutation rates is defined as the rate of non-germline variants detected in duplex consensus reads from a target-enriched WT sample, and was performed across Twist cfDNA v2 standard, native cfDNA, a competing DNA standard, and the Twist cfDNA v1 standard. The Twist Pan-Cancer v2 background error rate is similar to native cfDNA and lower than the previous v1 and competitor product due to a high-fidelity production process.
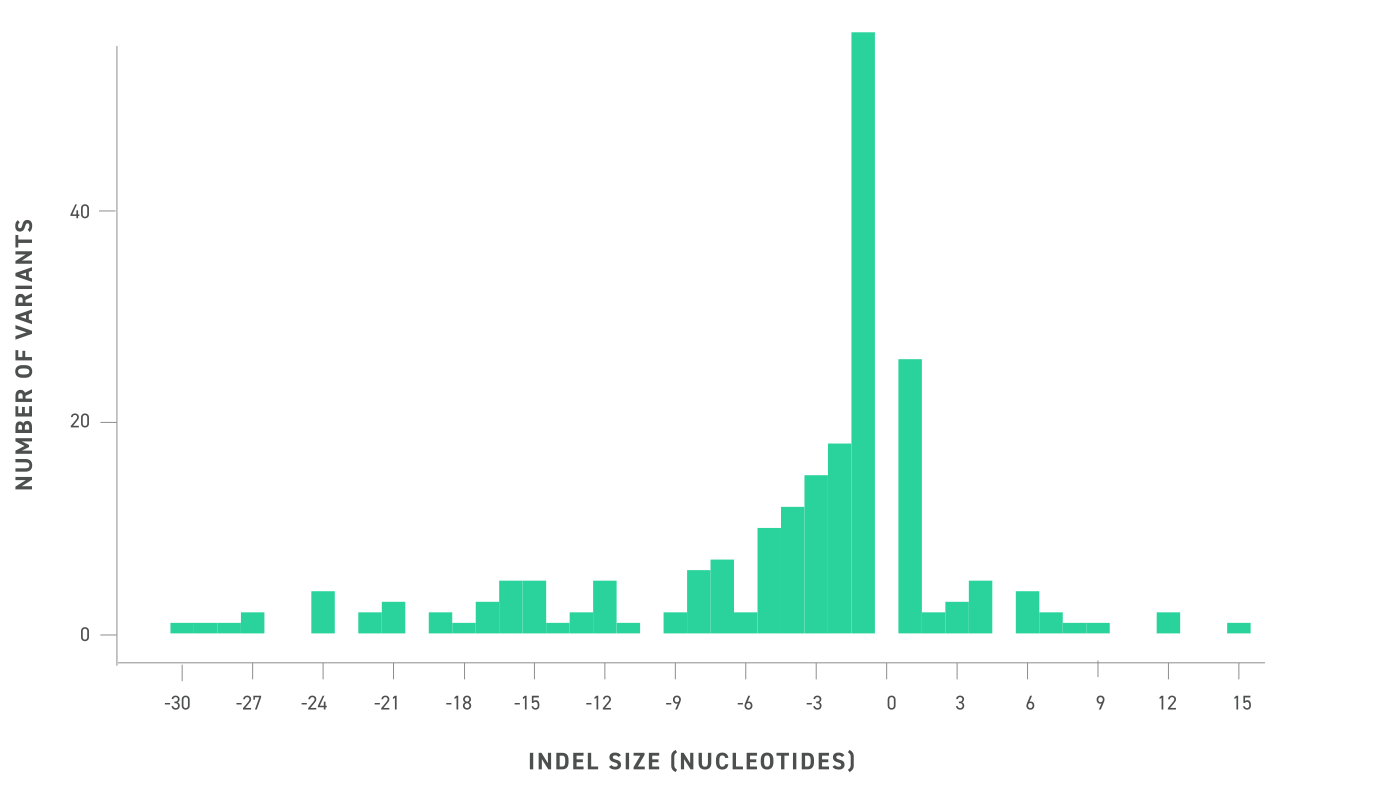
Visualization of the insertion or deletion size of the included INDELs in the reference standards via a histogram of binwidth 1, where negative numbers are deletions and positive numbers are insertions. SNVs are not shown. Included INDELs sample a wide range of sizes, allowing for broad analysis of INDEL detection performance. INDELs range from deletions of 30 bp and insertions of 15 bp.I was
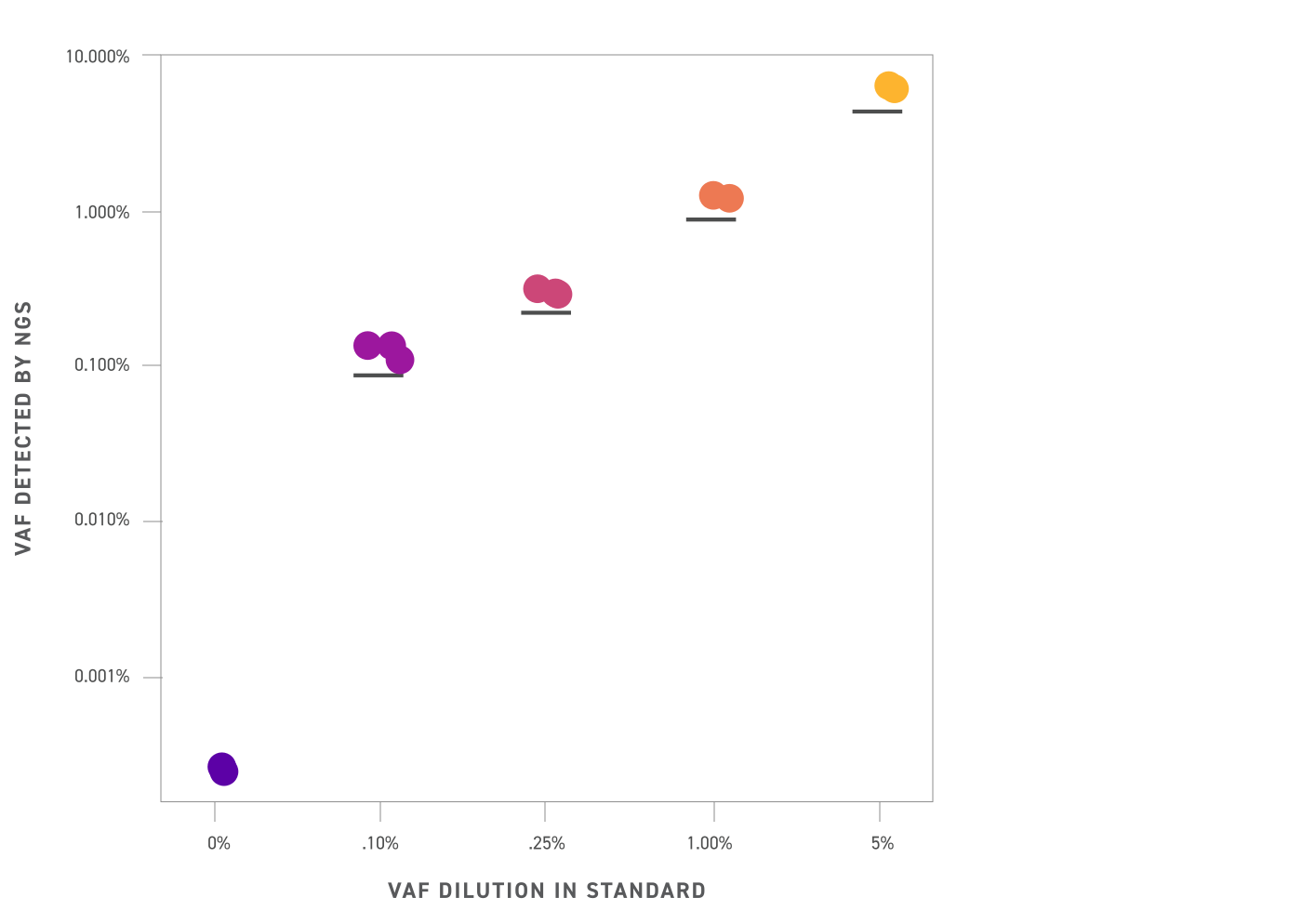
Average variant allele frequency (VAF) detected by NGS in different reference standard VAFs (shown on a log scale). The Twist cfDNA Pan-Cancer Reference Standards were captured with a custom panel targeting 215 single nucleotide variants (SNVs) found in the standards and sequenced to 80,000X raw coverage before UMI deduplication. Detected mean VAFs from NGS mirror closely the intended VAF across all levels tested, facilitating easy assay benchmarking.
DNA fragment analysis of the Twist cfDNA Pan-cancer Reference Standard v2, native cfDNA and competitor reference standards equalized for peak maximum height. Twist standards have a size distribution that emulates native cfDNA including a prominent mononucleosomal peak and dinucleosomal peak.

Variant DNA molecules tile across the genomic locus of the variant (purple dashed line with star) with extensive overlap, providing high coverage, fragment diversity, and location diversity of the variant site relative to the termini of the DNA molecule.

Analysis of background mutation rates is defined as the rate of non-germline variants detected in duplex consensus reads from a target-enriched WT sample, and was performed across Twist cfDNA v2 standard, native cfDNA, a competing DNA standard, and the Twist cfDNA v1 standard. The Twist Pan-Cancer v2 background error rate is similar to native cfDNA and lower than the previous v1 and competitor product due to a high-fidelity production process.

Visualization of the insertion or deletion size of the included INDELs in the reference standards via a histogram of binwidth 1, where negative numbers are deletions and positive numbers are insertions. SNVs are not shown. Included INDELs sample a wide range of sizes, allowing for broad analysis of INDEL detection performance. INDELs range from deletions of 30 bp and insertions of 15 bp.I was

Average variant allele frequency (VAF) detected by NGS in different reference standard VAFs (shown on a log scale). The Twist cfDNA Pan-Cancer Reference Standards were captured with a custom panel targeting 215 single nucleotide variants (SNVs) found in the standards and sequenced to 80,000X raw coverage before UMI deduplication. Detected mean VAFs from NGS mirror closely the intended VAF across all levels tested, facilitating easy assay benchmarking.
