As a rule of thumb, the easier cancer is to detect, the harder it is to treat.
Many tumors are detected only when seen (by imaging) or felt. For example, a doctor may feel a lump during a breast exam or discover a polyp during a colonoscopy. This means that in the fight against cancer, the race is on to identify ways to detect cancerous cells before the disease has significantly developed. A better understanding of the early warning signals and the development of tools to detect them promise to substantially improve the prognosis for new cancer patients and allow doctors to help those in remission by better detecting cancer the moment it returns.
But how can cancer be detected at its earliest stages when only a few cancer cells are present? One of the most promising potential answers involves screening our blood for cancer biomarkers.
Screening for cancer with liquid biopsies
At any given time, among the billions of blood cells, small fragments of DNA course through the bloodstream, originating from the normal turnover of cells. Cancer cells also shed DNA as they turnover, called circulating tumor DNA (ctDNA). Normal cell-free DNA (cfDNA) and ctDNA can be distinguished because the latter contains tumor mutations and epigenetic marks such as DNA methylation, enabling researchers to detect cancer by analyzing the DNA in a liquid (blood) biopsy.
🩸 What is a liquid biopsy?
A liquid biopsy is a simple blood test designed to evaluate the presence or extent of cancer. Used for detecting and managing many types of cancer, liquid biopsies typically involve identifying genetic biomarkers, such as tumor mutations or DNA methylation signatures, by sequencing the minute fraction of cell-free DNA that originates from tumor cells. Learn more about circulating tumor DNA and how researchers use liquid biopsies to analyze cancer’s methylation fingerprint here.
Liquid biopsies have the potential to detect cancer in real-time with non-invasive blood draws. But as easy as drawing blood is, identifying ctDNA in blood is non-trivial. CfDNA typically measures about 1-10 ng/ml in the blood. When cancer is present, ctDNA accounts for less than 1% of the total pool of cfDNA. If detected early enough, or if the sample comes from treated patients with minimal residual disease (MRD), this percentage may be lower than 0.1% or even 0.01%. This is the equivalent of trying to find a sewing needle dropped somewhere on a football field.
As you might expect, reliably finding such a needle is a significant challenge. To do so, significant research and advancements will be needed. One avenue of research involves the development of tools that allow scientists to home in on rare ctDNA fragments in a way that is robust across a wide range of sample qualities. Doing so requires high performance library preparation and target capture panels.
The Value of Preparation
The value of any next generation sequencing assay hinges on the quality and performance of the library preparation (library prep) workflow used. Library prep is the process of converting DNA (or RNA) into a format that’s compatible with next-generation sequencing technology. This typically involves fragmenting the DNA into short strands, repairing any single-strand overhangs, ligating adapters, and PCR amplification. With so few ctDNA fragments present in liquid biopsies, failing to convert even a single fragment could critically reduce overall assay sensitivity. Therefore researchers should reach for the best library prep kits.
This is where Twist’s cfDNA Library Preparation Kit can help. Relative to other commercially available kits, Twist’s cfDNA Library Preparation Kit produces libraries with greater yield, complexity, and uniformity, ultimately enabling researchers to build sensitive workflows for detecting rare variants in cell free DNA. Read more about this cfDNA library prep kit here.
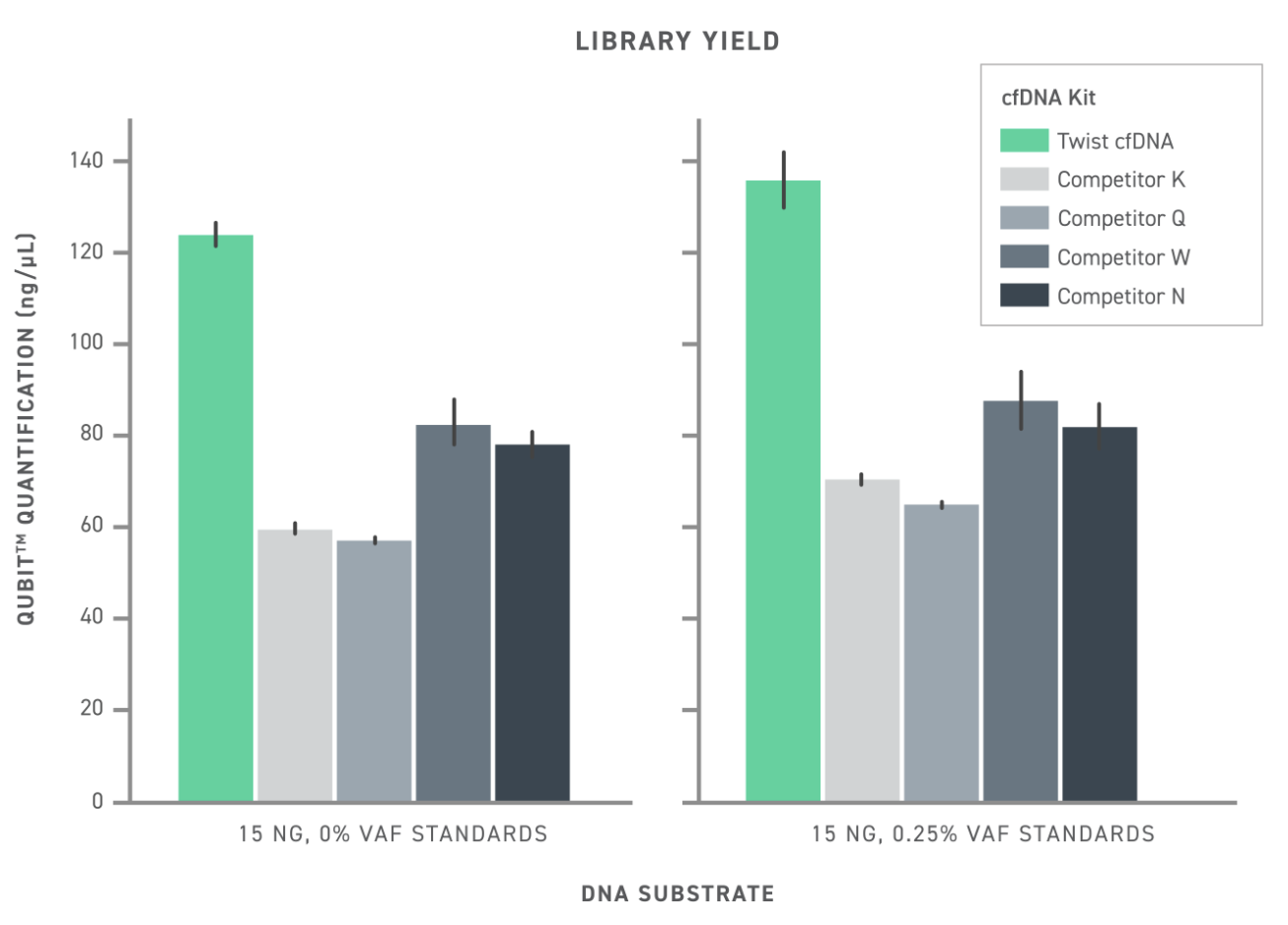
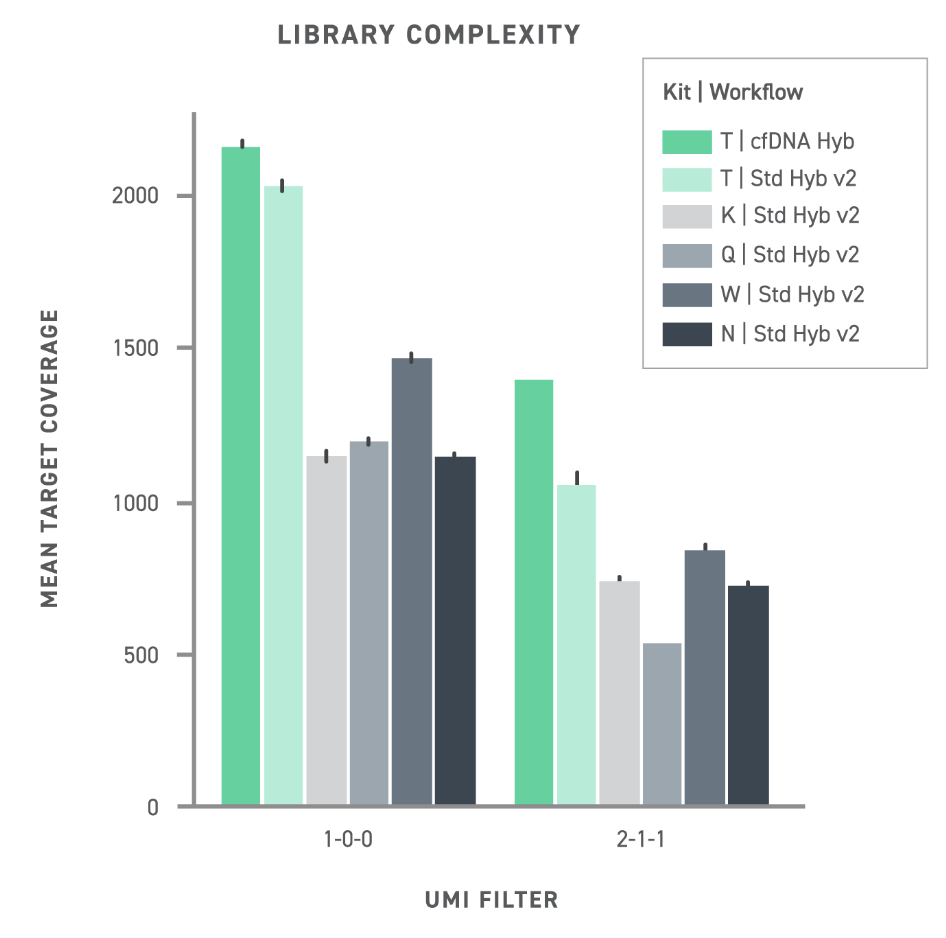
Comparison of commercial kits and Twist cfDNA Library Prep Kit (Twist cfDNA) performance. Library yield reflects the number of sequencing-ready fragments produced, reflecting the kit’s conversion efficiency. Library complexity refers to the number of unique sequences detected as singlets (1-0-0) or with complementary strand pairing (2-1-1).
How researchers study fragments of cancer DNA
If ctDNA is a sewing needle lost on a football field, target capture sequencing is the magnet researchers use to find it. By selectively pulling out the molecules of interest (ctDNA) and tossing aside useless sequences (normal cfDNA), target enrichment not only increases the effective concentration of ctDNA in a sample, significantly improving the search effort, it also cuts the cost of sequencing the sample.
Twist offers a variety of target capture panels, including Twist Custom Panels, which users can design and build to cover a wide range of panel sizes (100 to >1M probes per panel), target regions, tiling, and GC content requirements. Twist Bioscience also offers extensive support for custom panel design to ensure the maximum efficacy of any custom panel. Read our recent blog to learn more.
For a clinical sample looking for a specific type of cancer, like colorectal cancer, before a doctor informs their patient that they have cancer, they would want to be very confident that the results they see truly mean that their sample shows cancer and not just a benign variation in their genome.
For any assay to be trusted, validation is essential. Typically, a researcher will validate that the sample contains cancerous DNA using controls—fragmented DNA from cancer cell lines or modified reference samples. However, such approaches for sample creation can be costly, labor-intensive, and error-prone. In applications requiring high sensitivity—such as studying ctDNA—small inaccuracies in the control samples can lead to erroneous performance results. The Twist Pan-Cancer control is precisely synthesized to contain common cancer variants, as well as a near-perfect 1:1 map of the human background to help researchers develop accurate, analytically validated assays for cancer detection.
Cancer's signature is written in mutations and epigenetic patterns
When building assays to study specific cancer types, researchers must also decide which DNA signatures they would like to detect. Tumor mutations are the most obvious biomarkers, as they can be readily detected by sequencing. In addition, researchers are studying the potential of using the complete complement of mutations found in a tumor, known as the tumor’s mutational burden, to predict a tumor’s responsiveness to therapy. Target enrichment panels such as the Twist Human Core Exome or Twist Exome 2.0 offer deep, comprehensive, and uniform coverage of the human exome, making it possible to detect these tumor-specific signatures with minimal sequencing.
DNA Methylation As A Cancer Biomarker
Obvious as they are, tumor mutations are not the only signposts of cancer, nor are they necessarily the best marker for every application. Tumor mutations can sometimes be challenging to separate from those that accumulate during normal aging. Moreover, mutational signatures evolve as tumors grow, and they also don’t usually reveal a tumor’s origin in the body.
In the search for clearer answers, researchers are beginning to look into the possibility of using tumor DNA methylation patterns as a more sensitive biomarker for cancer detection. Compared to mutational signatures, DNA methylation patterns tend to be more disease- and tissue-specific, and they can often be more readily detectable at the earliest stages of disease. Consequently, researchers want to know if methylation patterns in ctDNA can be used to both detect cancer and identify precisely which tissue it is derived from.
However, detecting DNA methylation patterns comes with its own set of obstacles. The process involves converting unmethylated cytosines to thymines and distinguishing them from methylated cytosines. But this conversion step invariably reduces the complexity of the sequencing library, making target enrichment more challenging. What’s more, conventional methylation sequencing protocols that use bisulfite salts typically require large sample inputs to account for loss during conversion. Not great for ctDNA.
To enable sensitive methylation sequencing in ctDNA research, Twist developed the Twist NGS Methylation Detection System and Twist Custom Methylation Panels. The system uses an enzymatic conversion process that minimizes DNA damage and loss during library preparation. Methylation conversion can be paired with Twist hybrid-capture panels, both custom methylation panels and a broader off-the-shelf solution. In addition to possessing the same fidelity, uniformity, and flexibility as Twist Custom Panels, Twist Custom Methylation Panels are custom-designed to capture all four possible sequences at every targeted site: methylated, unmethylated, sense, and antisense. Similarly, Twist’s Human Methylome Panel is designed to capture methylated bases across a broad swath of the human genome, encompassing more than 84% of CpG islands.
Read our blog to learn more about ctDNA and enzymatic methylation sequencing, or listen to how other researchers use Twist methylation sequencing products to research novel methylation signatures in tumors and the potential of detecting cancers below the current limit of detection.
Mutations or methylation, liquid biopsy or archival tumor sample, Twist’s ever-expanding toolkit of NGS solutions is helping to transform the face of cancer research with expertly designed and precisely synthesized target enrichment panels and optimized library preparation reagents. The combination of the two offers unparalleled detection sensitivity, perfect for low-input applications like those used to reasearch early cancer screening and MRD monitoring.
Contact our team to discuss how Twist can help power your cancer research.