How Llama Antibodies May Help us Fight Coronavirus

A study by Wrapp et al. just published in Cell shows antibodies from llamas block SARS and SARS-CoV-2, the virus that causes COVID-19 (Wrapp et al., 2020). While this may pave the way for a potential treatment for the global coronavirus pandemic, it has left many people with a curious question:
Why llama antibodies?
Camelid Immune Systems are Unusual
Legend has it that in the late 1980s, during a lab skills lecture at the Free University of Brussels, students were tasked with extracting antibodies from human blood serum. Bored with this routine experiment, the students started rummaging through the freezer for more interesting samples. The students chanced upon a sample of camel blood, and what they then found changed the immunotherapeutic field forever. Alongside the expected immunoglobulins were unexpectedly smaller versions that did not correspond to anything known to science at the time. Researchers investigated more deeply, and found that all camelids (including dromedaries, llamas and alpacas) have unusual immune systems (Gross, 2000).
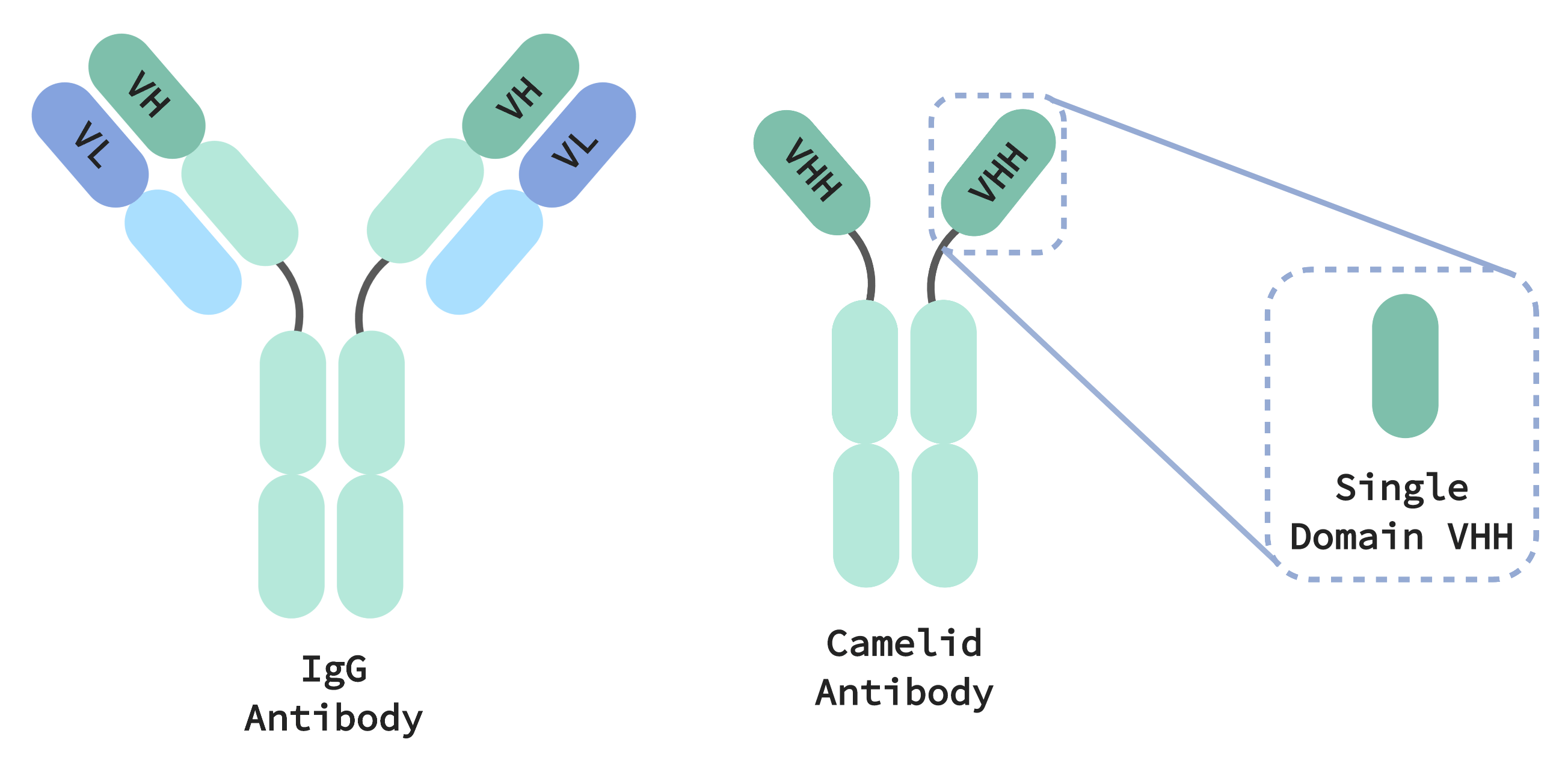
Camelid immune systems generate smaller antibodies that are both simpler and more compact than the immunoglobulin G (IgG) antibodies found in all mammals. IgGs are made up of two different protein chains, heavy and light, that must pair together and cooperate to specifically recognize a target. This specific targeting affords our immune systems “memory”, allowing it to selectively and precisely eliminate pathogenic threats. Target recognition by camelid antibodies, on the other hand, requires just a single domain found on a single protein chain, called a VHH (equivalent to the variable region of a heavy chain in IgG). With VHH-based antibodies able to exhibit pharmaceutically relevant properties comparable to IgGs, they are a promising therapeutic with several advantages over their bulkier, more complex, counterparts.
For one, the small size of camelid antibodies means they can squeeze into spaces and bind or block to parts of molecules that would otherwise be inaccessible to human IgG antibodies. They are also more thermally- and chemically-stable making VHH-based therapeutics good candidates to address respiratory infections, administered by inhaler directly to the respiratory tract where the infection is concentrated. At the same time, these properties all afford the molecules a long shelf life.
Manufacturing of VHH-based therapeutics is also simpler. IgG antibodies are encoded by two genes which must be stored, transfected and expressed as a specific pair in order to function. Multiply that by thousands of candidate antibodies, and IgG manufacture very quickly becomes an incredibly logistically complex process. On the other hand, once a candidate VHH antibody is isolated it only needs to be expressed by a single gene, meaning much less overhead is required to track, store and perform experiments on these molecules. Expression of just the VHH domain can also be performed, producing therapeutic molecules one tenth the size of a conventional IgG antibody.
Llama Antibodies for Coronavirus Therapeutics
2020 has been dominated by the COVID-19 pandemic. There are two key molecules at play during the initial binding of the SARS-CoV-2 virus to a human cell: the virus’ receptor domain binding S (“Spike”) protein, and the host cell’s angiotensin converting enzyme 2 (ACE2). Spike protein binds tightly to ACE2, allowing the virus to penetrate into the host cell. Blocking the interaction of these two proteins is an important therapeutic target. In theory, an antibody tightly binding either protein would neutralize coronavirus particles, halting the virus from entering new cells.
To discover Camelid antibodies against SARS-CoV-2, Wrapp et al. immunized llamas with a stabilized form of the closely related SARS spike protein. Among the VHHs generated by the llama immune systems was a candidate molecule which bound tightly to the Spike protein and blocked SARS infection.
As SARS is closely related to SARS-CoV-2, researchers then tested the antibody’s ability to cross-react with the equivalent SARS-CoV-2 spike protein. While the initial interaction was weak, a stronger version was created by fusing it to part of a human IgG. This fusion molecule was able to effectively neutralize SARS-CoV-2.
Given the paucity of both prophylactic and therapeutic treatments currently available to treat the coronavirus epidemic, it is hoped that these camelid-based therapeutics will contribute to both research into, as well as treatment for, COVID-19.
Expert Opinion
"At Twist, we have a deep appreciation for llamas as their immune system contains antibodies that only have a heavy chain. These VHH or single domain antibodies are small, easy to produce, and bind to unique sites, or epitopes, on disease targets,” remarks Aaron Sato, Ph.D., CSO of Twist Biopharma, a division of Twist Bioscience.
“With these molecules in mind, we developed a panel of synthetic VHH antibody phage display libraries derived from natural llama sequence diversity,” Dr. Sato points out. “Using these libraries, you can identify VHH antibodies without immunizing llamas and reduce time to discovery!"
Recently, the Twist Biopharma team led by Dr. Sato utilized their llama-inspired synthetic single domain libraries to discover VHH antibodies to ACE2 in less than 6 weeks. Many of these VHH hits showed potent binding to the receptor and inhibited ACE2 from binding to SARS-CoV-2 S1 protein. This case study exemplified the power of these libraries to quickly address another important therapeutic target for the COVID-19 pandemic.
Click here for more on Twist Biopharma’s synthetic VHH antibody libraries, and how they have been used to generate candidate COVID-19 therapeutics.
References
Wrapp et al., Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid
Antibodies, Cell (2020), https://doi.org/10.1016/j.cell.2020.04.031
Michael Gross, One reason to get the hump, The Guardian (2000), accessed 26th May 2020, https://www.theguardian.com/science/2000/sep/14/technology1
Cover Photo by Jessica Knowlden on Unsplash
What did you think?
Like
Dislike
Love
Surprised
Interesting